Research |
We study synthesis, characterization, and properties of various nanoparticles and nanostructured hybrid materials for catalyitc, biomedical and energy applications. Our reseacrh is published promptly. Some articles have been chosen for journal cover pages (see below).
 |
| 
 |
| 
We are interested in synthesis and study of various functionalized nanoparticles and polymer based nanocomposites. They represent an exciting area of nanoscience and nanotechnology allowing unique properties such optical, magnetic, catalytic, sensing etc. and applications of these materials for energy, in biomedical fields and in catalysis.
Functionalized Magnetic Nanoparticles
Magnetic nanoparticles are of increased importance for various biomedical applications such as enhanced contrast agents for magnetic resonance imaging (MRI) and for hyperthermia cancer treatment. Because magnetic properties are size dependent, monodisperse magnetic nanoparticles are preferred.
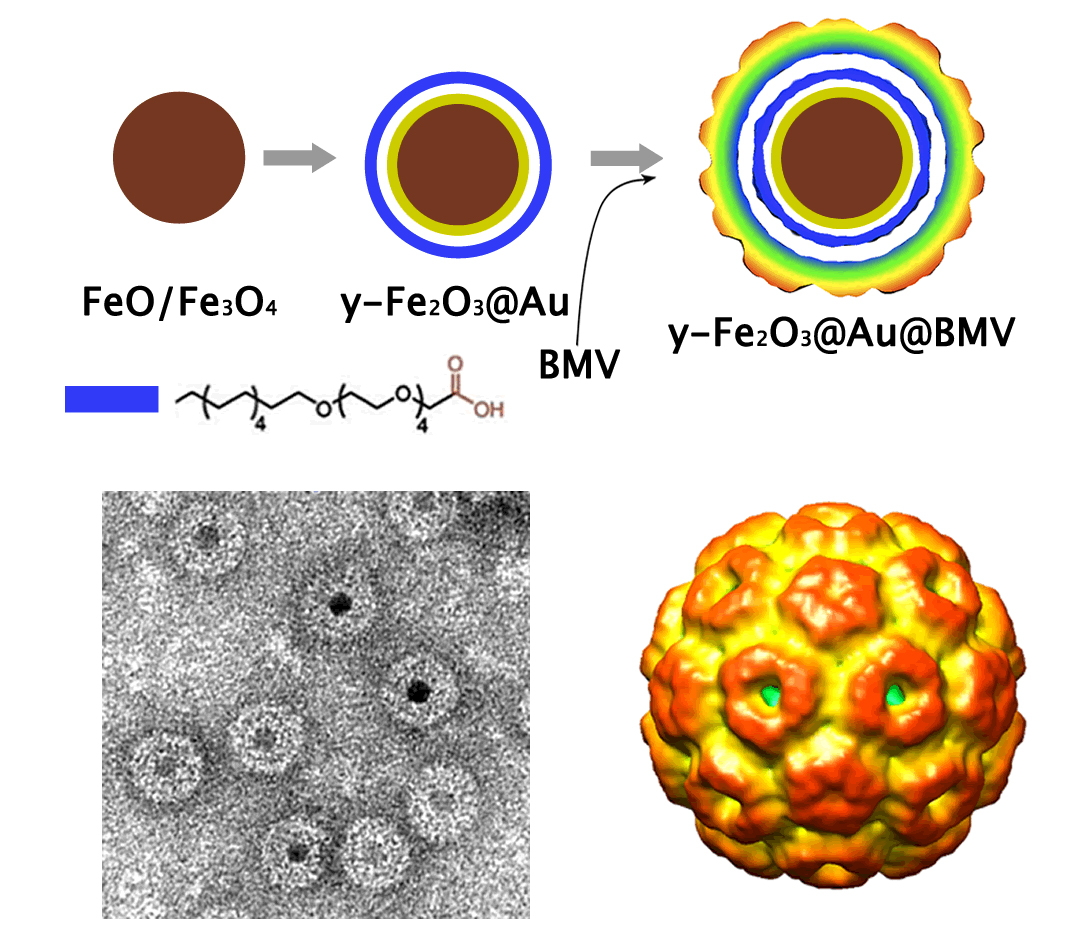
We synthesize monodisperse magnetic nanoparticles of different shapes, sizes and composition via organometallic routes. The proper functionalization can make these particles hydrophobic or hydrophilic and can determine the interaction forces between the particles. As major means of functionalization we use hydrophobic interactions of the nanoparticle protective layer with functionalized lipids or amphiphilic copolymers, covalent interaction of the particle surface with functional silanes, or ligand exchange. The negatively charged water soluble nanoparticles (left, below) can be additionally coated with virus protein forming virus-like nanoparticles (VNPs, right, below).
We also developed VNPs utilizing gold-coated iron oxide nanoparticles as cores and capsid protein of brome mosaic virus (BMV) or hepatitis B virus (HBV) as shells. Further, utilizing cryo-electron microscopy and single particle methods, we were able to show that the BMV coat on VNPs assembles into a structure very close to that of a native virion (see below). This is a consequence of an optimal iron oxide NP size (~11 nm) fitting the virus cavity and an ultrathin gold layer on the maghemite cores, which allows for utilization of SH- (CH2)11-(CH2-CH2-O)4-OCH2-COOH as capping molecules to provide sufficient stability, charge density, and small form factor. MRI studies show unique relaxivity ratios that diminish only slightly with gold coating. A virus protein coating of a magnetic core mimicking the wild-type virus makes these VNPs a versatile platform for biomedical applications.
Magnetically Recoverable Catalysts
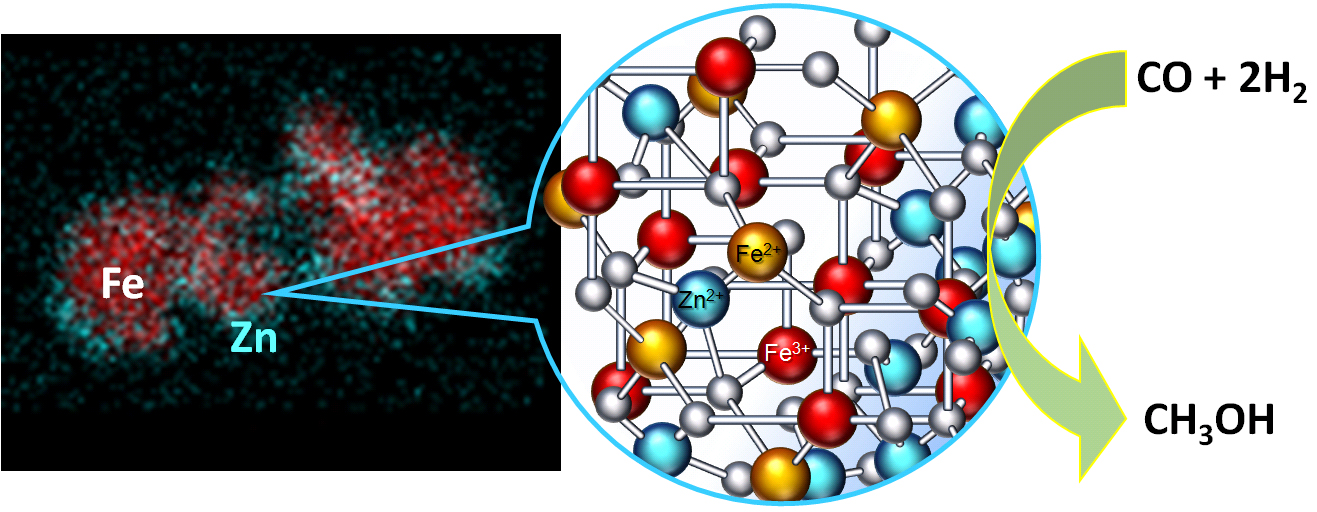
Another avenue for magnetic nanoparticles is the development of magnetically recoverable catalysts. In this case, magnetic nanoparticles can be synthesized in the presence of functional capping molecules such as polyphenylenepyridyl dendrons or thermally stable polymers (collaboration with Dr. Zinaida Shifrina's group from A.N. Nesmeyanov Institute of Organoelement Compounds of Russian Academy of Sciences, Moscow, Russia) bearing functional groups. Such dendrons/polymer shells can stabilize catalytic species on top of magnetic nanoparticles, and allow for easy magnetic separation. The graphic below shows the STEM EDS map and a schematic representation of Zn-containing magnetic oxide nanoparticle stabilized by polyphenylquinoxaline. The catalytic testing in syngas conversion to methanol demonstrated outstanding catalytic properties of Zn-containing magnetic oxides, whose activities are dependent on the Zn loading. Repeat experiments carried out with the best catalyst after magnetic separation showed remarkable catalyst stability even after five consecutive catalytic runs.
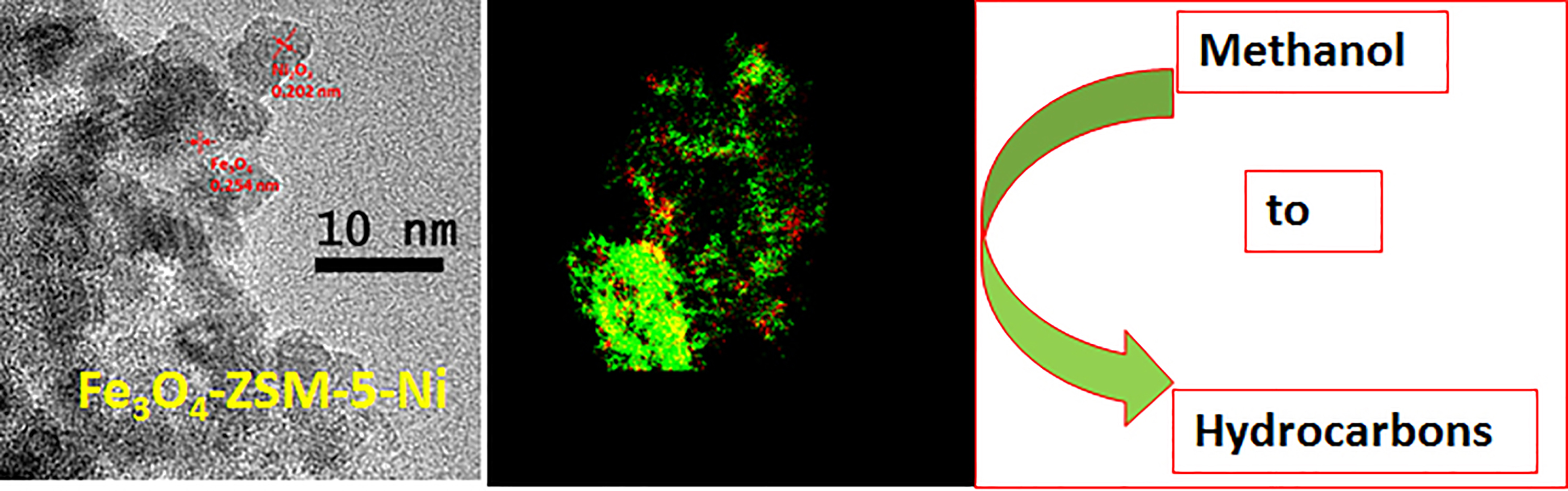
The other approach to magnetically controllable catalysts is the development of hierarchical zeolites (ZSM-5) containing both iron oxide and nickel oxide nanoparticles. Modifying the iron oxide (magnetite) amounts, we are able to control the catalyst activity and the product distribution in the metanol-to-hydrocarbons process, allowing one to utilize renewable sources of both value-added chemicals and fuels. Addition of Ni species to Fe3O4-ZSM-5 leads to the formation of mixed Ni oxides (NiO/Ni2O3) positioned either on top or next to Fe3O4 nanoparticles. This modification allowed us to significantly improve the catalyst stability due to diminishing coke formation and disordering of the coke formed.